‘Eye-saving’ oral UM treatment
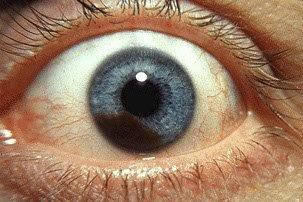
Interim results from the ongoing phase 2 Australian trial of darovasertib, an oral neoadjuvant/adjuvant uveal melanoma (UM) treatment, showed 75% of treated patients were spared enucleation, reported the drug’s US developer, Ideaya Biosciences.
Fifteen patients with localised UM scheduled for enucleation were treated with darovasertib 300mg twice daily. An initial safety cohort of three patients was treated for one month, with the remaining 12 patients treated for up to six months prior to their primary intervention (enucleation, plaque brachytherapy or external beam radiotherapy (EBRT)) across three Australian centres. The results were presented at the American Society of Clinical Oncology (ASCO) 2024 annual meeting by Associate Professor Anthony Joshua, lead principal investigator at St Vincent’s Hospital in Sydney, who reported that, of those 12, nine (75%) had an eye saved and eight (67%) had greater than 30% tumour shrinkage after six months.
These data highlight the potential of darovasertib as a neoadjuvant treatment to provide meaningful tumour shrinkage in patients with ocular tumours and spare patients from enucleation, with a manageable adverse-event profile, said Dr Darrin Beaupre, Ideaya’s chief medical officer. "We are targeting a Type C meeting with the FDA in H2 2024 for guidance on a potential registrational trial in neoadjuvant UM, and we are encouraged by the rapid enrolment and preliminary clinical efficacy and safety observed from Ideaya's phase 2 neoadjuvant UM trial."